Abstract
Introduction
The impact of new therapies for CLL on health-related Quality of Life (HRQoL) is unclear in the real-life setting. We aimed to evaluate the impact of Ibrutinib (Ibr) monotherapy in the QoL of patients (pts) with CLL based in EuroQol (EQ-5D-5L) and FACIT-fatigue (FACIT) questionnaires assessment from baseline to 1 year of treatment.
Methods
This prospective observational study (NCT04016636) was conducted across 5 sites in Argentina. Key eligibility criteria: CLL adult pts with indication to start single-agent Ibr therapy, in the 1st-line or relapsed setting. QoL was assessed with FACIT and EQ5D5L at baseline, 1, 3, 6 and 12 months (mo) during treatment. Minimally clinically important differences (MID) was defined as "the smallest difference in score perceived as beneficial" (Cella D et al. 2002). MID was=3 points for FACIT-score, =0.063 for EQ5D5L index-score (EQ5D5L-IS), =7 for EQ5D- visual analog scale (VAS), and =1.5g/dl hemoglobin (Hb) variation.
Main outcome: determine improvements in QOL at 12 mo of treatment according to MID through mean (M) differences (one-tailed test for paired data).
A sensitivity analysis was done excluding the quartile with least impairment at baseline (EQ5D-VAS >85, FACIT >48, Hb >12.7, ED5D5L-IS 1).
Secondary Outcome Measures: Number of pts with improvement in the EQ-5D-5L domains by analyzing variation in each domain.
Exploratory endpoint: Determine association between baseline Hb levels and FACIT change from baseline.
Results
From 48 pts who signed informed consent, 46 (95.8%) provided baseline responses and had ≥1 post-baseline value. Overall compliance rates on the PRO were ≥95%.
Median (Md) age was 73 years (SD 10.8). Ibr (single agent) was 1st-line therapy in 87%, Md duration therapy at the cutoff was 13 mo (range 8-28) and still ongoing. Baseline QoL scores were indicative of a moderate fatigue level and impaired functioning: M baseline FACIT 39.3 (standard-deviation [SD] 12.4); M EQ5D5L-IS 0.90 (SD 0.12); M EQ5D-VAS 70.17% (SD 20.49) and M Hb level 11.1 g/dl (SD 2.14).
At the 1st mo, improvement per MID threshold (MID-T) was met in 28.3% pts with FACIT, 26% with EQ5D-VAS, and 23.9% with EQ5D5L-IS. At 3 mo MID-T was met in 43.5% pts with FACIT, 32.6% with EQ5D-VAS and 32.6% with EQ5D5L-IS. At 6 mo MID-T was met in 52.3% pts with FACIT, 45.5% with EQ5D-VAS and 27.3% with EQ5D5L-IS. At 12 mo MID-T was met in 52.4% pts with FACIT, 45.2% with EQ5D-VAS and 26.2% with EQ5D5L-IS.
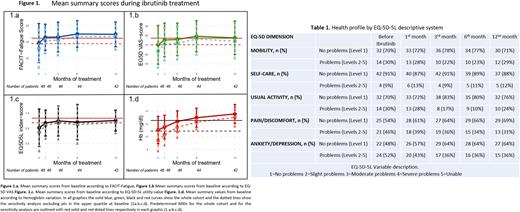
In the analysis of the whole cohort M differences at 1, 3, 6 and 12 mo of treatment were: with FACIT MD 3.56, 3.73, 5.7 and 6 respectively (p<0.001); with EQ5D-VAS MD: 5.2, 5.6, 9.7 and 9.3 respectively (p<0.06); with EQ5D5L-IS MD: 0.033, 0.04, 0.04 and 0.048 respectively (p=0.46). (Figure 1a,1b,1c).
The M of FACIT reached the MID after 1 mo, the M of EQ-5D-VAS reached the MID after 6 mo and the M of Hb increase >1.5g/dl was reached after 6 mo of treatment with Ibr. Observing positive changes from baseline over time in M scores on the 3 groups.
Hb improvement (≥1.5 g/dl) during treatment was achieved by 13% at 1 month [MD 0.47]; 24% at 3mo [MD 1.33]; 20.4% at 6mo [MD 1.98]; and 9.5% at 12mo of treatment [MD 2.51 (p=0.001)]. (Figure 1d).
Excluding the quartile with least impairment at baseline, MID was achieved earlier: the M of FACIT after 1 mo, the M of EQ5D-VAS after 1 mo and the M of Hb variation >1.5g/dl after 3 mo of treatment with Ibr. In addition, in this subgroup MD were more pronounced: FACIT 4.9, 5.1, 7.6, 8.5 at mo 1, 3, 6 and 12 respectively (p<0.001), EQ-5D-VAS 7.9, 8.9, 13.1, 13.1 at mo 1,3,6 and 12 respectively (p<0.03), Hb increase 0.6, 1.6, 2.3, 2.8 at mo 1, 3, 6 and 12 respectively (p<0.04) and EQ5D5L-IS 0.045, 0.066, 0.05, 0.064 at mo 1, 3, 6 and 12 respectively (p<0.46) (Figure 1a, 1b, 1c, 1d dotted line). Suggesting that pts with greater extent of QoL impairment at baseline experienced greater and more rapid improvement in QoL scores.
Results from the EQ5D5L descriptive system are presented as a health profile in Table 1.
We observed that pts with baseline Hb ≤11g/dl achieved higher FACIT MD after 6 mo of treatment (p= 0.028); suggesting more improvement in those who start treatment with a lower Hb.
Conclusion
This is to our knowledge the 1st real-world study in Latam designed to evaluate HRQoL with Ibr as monotherapy. We observed evidence of positive HRQoL effects particularly in fragile pts with greatest extent of impairment at baseline. Results from longer follow-up are needed to evaluate the impact on HRQoL with extended Ibr therapy.
Disclosures
Pavlovsky:Novartis, Pfizer, BMS, Pint Pharma: Speakers Bureau; Novartis, Pfizer: Membership on an entity's Board of Directors or advisory committees. Pavlovsky:AstraZeneca: Other; Janssen: Other: Conference; AbbVie: Other: Conference; Janssen: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Raffo: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal